How many colours do you see when you hear the words “coral reef”? We imagine bright yellows, marvellous reds, gorgeous magentas, deep purples and marvellous oranges, but how close is that to the truth?

Figure 1. Bleached coral. Source: Recording and revealing the third global bleaching event, 2017, photograph, XL Catlin Seaview Survey
In reality, many coral reefs nowadays do not have such colours. Many are now as dull as sand. Some white, some grey, you might spot a slight shade of diluted lavender, but overall dead and lifeless. The sights we dream of when we think of going scuba diving someplace far and tropical, now look corporate and even outright terrifying.
What happened? Are we just shown a fantasy in movies, the beautiful colours all fake? Are they supposed to be this way? Actually, healthy coral reefs look even more majestic than the ones we imagine, but many nowadays face coral bleaching, a process where environmental stressors suck out the colourful, energy-producing algae inside them, leaving them an expressionless white. [1] One of the environmental stressors that these algae face is ocean acidification, which is a growing problem that is leaving devastating impacts on marine ecosystems and species and causing irreversible damage to habitat quality.
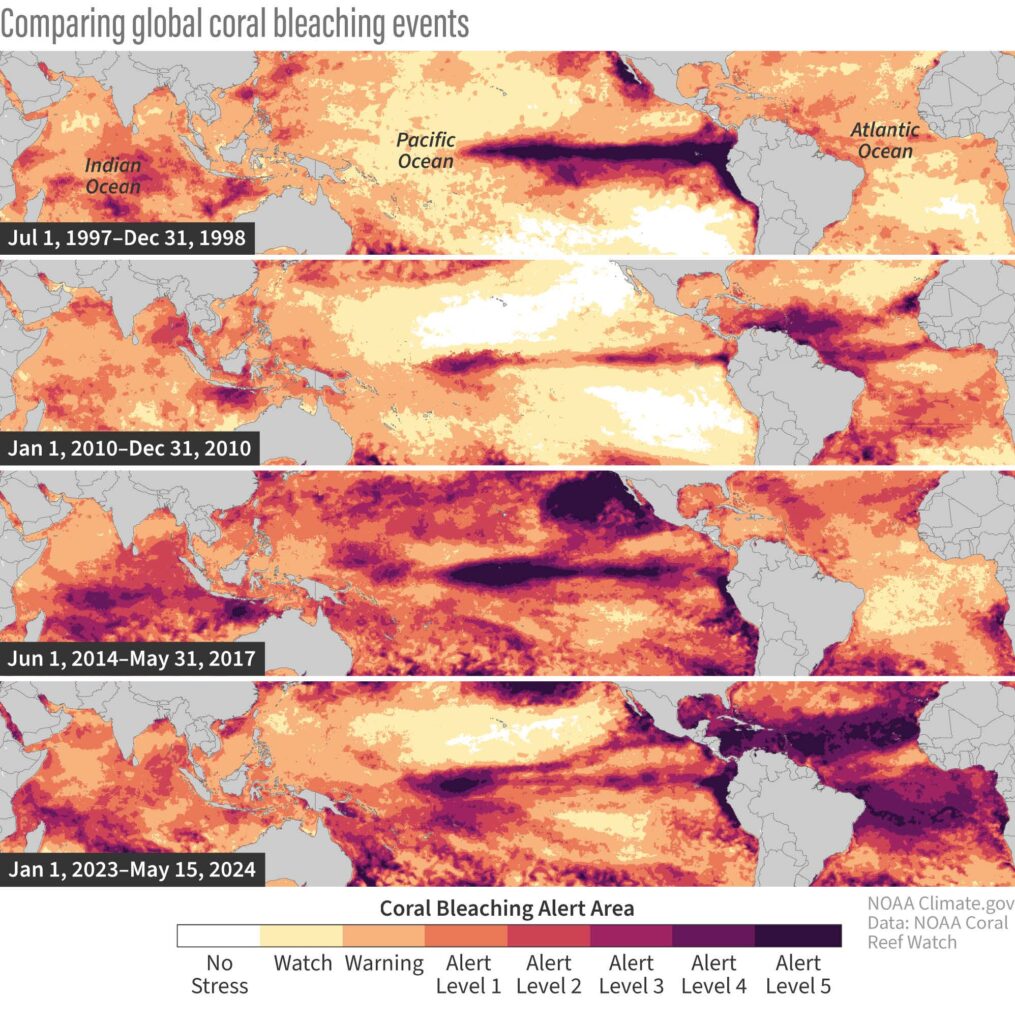
Figure 2. Comparing the Extent of Global Coral Bleaching Events in 1998, 2010, 2014–2017, and 2023–Present Using NOAA’s Coral Reef Watch Bleaching Alert Levels. The maps show a drastic increase in severity of coral bleaching events across the globe. Source: Comparing global coral bleaching events, 2024, image/png, Climate.gov Media
What is ocean acidification?
Many of us are familiar with the concept of global warming, the gradual increase in global temperatures caused by greenhouse gases in the atmosphere, [2] namely carbon dioxide (or CO₂) [3], but this increased concentration of CO₂ can have other consequences.
Our oceans absorb about 26% of the carbon dioxide released into the Earth’s atmosphere. [4] As a result, the pH of the surface of ocean waters has fallen by 0.1 units. Although it sounds like a tiny, miniscule change, it represents approximately a 30% increase in acidity, which has disastrous effects on the ocean’s overall health. The ability of some fish to detect predators and find habitats is decreased in more acidic waters, [5] and marine organisms such as oysters, their shells and skeleton even dissolve. [6] But how is all this affected by CO₂ in the atmosphere?
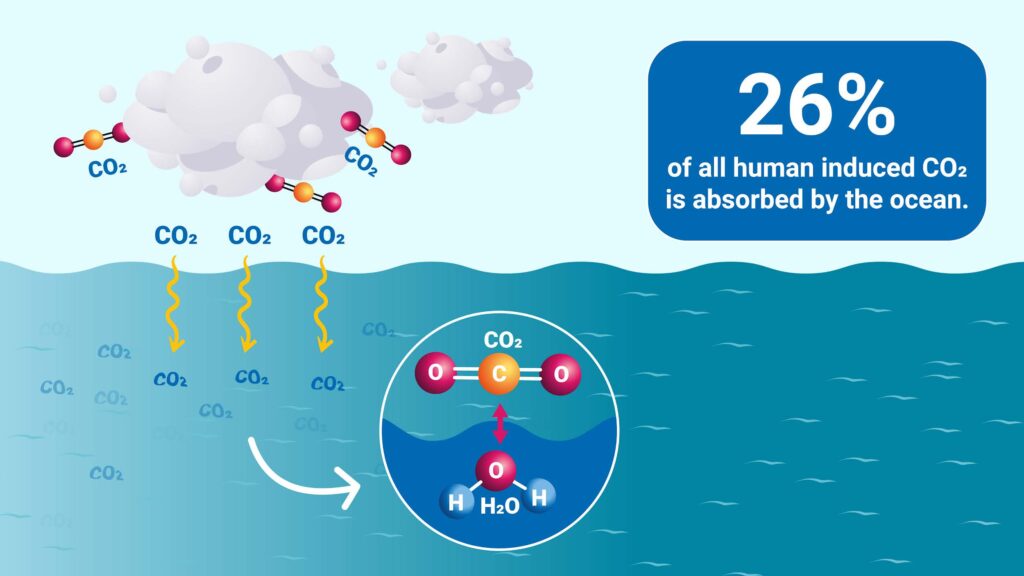
Figure 3. How CO₂ emissions result in ocean acidification. Source: A. Vargas Terrones/IAEA, 26% of all human induced CO₂ is absorbed by the ocean, 2022, graphic, How Carbon Emissions Acidify our Oceans
When carbon dioxide (CO₂) is absorbed by water (H₂O), a series of chemical reactions occur resulting in an increased concentration of hydrogen ions. First, they combine to form carbonic acid (H₂CO₃). Because the acid is weak, one of the hydrogen atoms snaps off, resulting in two parts: a positive hydrogen ion (H⁺) and a negative bicarbonate ion (HCO₃⁻). These hydrogen ions cause the pH level to decrease (the pH level is an inverse of the presence of hydrogen ions, so the higher the pH, the lower the hydrogen ion count, and vice versa), and as a result, the water becomes more acidic.
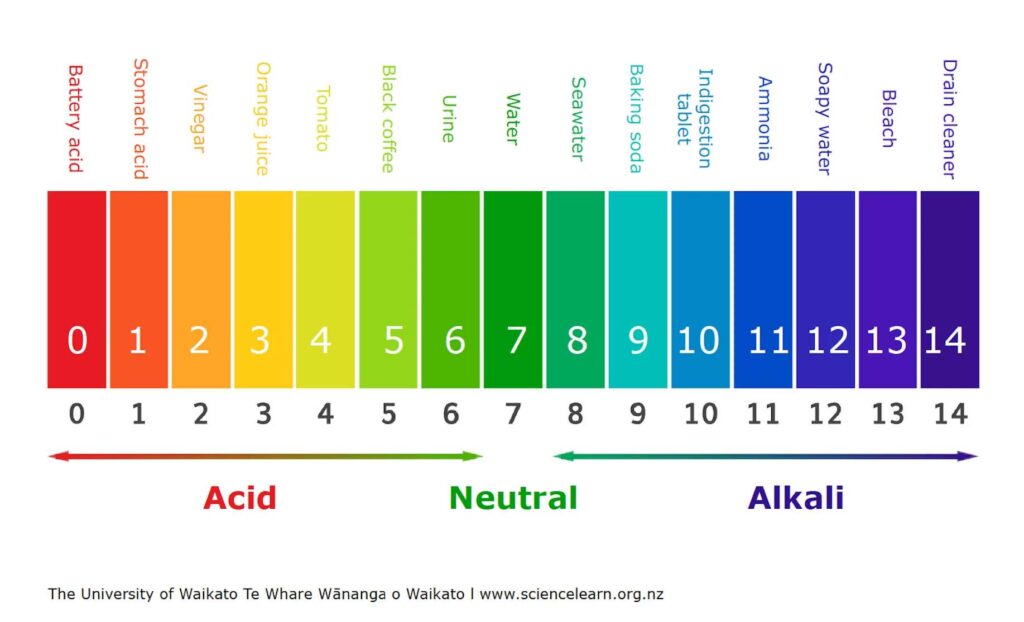
Figure 4. the pH scale. 7 units is considered neutral, anything lower is acidic and anything
higher is alkaline. Source: pH scale, 2021, image, Science Learning Hub – Pokapū Akoranga Pūtaiao, The University of Waikato Te Whare Wānanga o Waikato
Currently, the ocean’s pH average is 8.1, which is expected to fall to 7.8 by the end of this century, a number not seen since the middle Miocene 14-17 million years ago, a nightmare period when the earth was several degrees warmer, a major extinction event was occurring [5] and there were high atmospheric CO₂ concentrations [7] (hence the acidic pH level).
Suffering calcifying organisms
Besides coral bleaching, another terrifying result of ocean acidification is the fate of calcifying organisms, organisms that build shells, skeletons and other structures by combining the carbonate ions (CO₃²⁻) present in the ocean with calcium (Ca). However, as ocean acidification increases, these carbonate ions bond with the hydrogen ions (H⁺) instead, so fewer carbonate ions are available for organisms to build and maintain their structures, which can even dissolve if the pH levels are too low. [5]
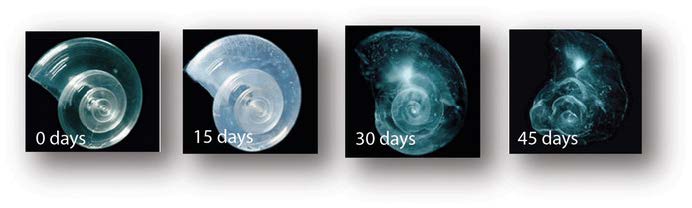
Figure 5. Progressive dissolution of a pteropod shell under acidic conditions. The shell shows
increasing disintegration over 45 days when placed in seawater with pH and carbonate levels projected for the year 2100. Source: © David Liittschwager/National Geographic Stock, Pteropod shell experiment, 2005, photograph, National Geographic Images
Can ocean acidification be fixed?
Despite many organisms suffering at the hands of ocean acidification, some are benefitting from it. Algae and seagrasses can benefit from higher CO₂ concentrations in the ocean as they need it for photosynthesis, and there are some ongoing studies to see if growing more seaweed can help slow ocean acidification.
Some studies show that ocean acidification can also be a result of plastic pollution in the ocean due to the accumulating bacteria and microorganisms on the plastic garbage, so fixing the plastic problem can also help reduce the effects of ocean acidification.
In conclusion, our planet’s vibrant coral reefs are fading into pale, lifeless structures due to the growing crisis of ocean acidification. Mainly driven by carbon dioxide emissions, ocean acidification is harming the ocean as a whole, affecting many of its ecosystems, habitats and species. Tackling this problem will require urgent action —reducing carbon emissions, addressing plastic pollution, and exploring innovative solutions like seaweed farming. If we act now, there is still hope to preserve the breathtaking underwater worlds we dream about.
References
- Margaux Monfared. 2025. “84% of the World’s Coral Reefs Impacted in the Most Intense Global Coral Bleaching Event Ever | ICRI.” ICRI. April 23, 2025. https://icriforum.org/4gbe-2025/.
- Morgan, James. 2022. “Causes of Global Warming.” WWF Australia. 2022. https://wwf.org.au/what-we-do/climate/causes-of-global-warming/.
- MacMillan, Amanda, and Jeff Turrentine. 2021. “Global Warming 101.” NRDC. NRDC. April 7, 2021. https://www.nrdc.org/stories/global-warming-101#causes.
- Tarakanov, Vladimir. 2022. “How Carbon Emissions Acidify Our Ocean.” Www.iaea.org. December 15, 2022. https://www.iaea.org/bulletin/how-carbon-emissions-acidify-our-ocean.
- NOAA. 2025. “Ocean Acidification.” National Oceanic and Atmospheric Administration. U.S. Department of Commerce. February 25, 2025. https://www.noaa.gov/education/resource-collections/ocean-coasts/ocean-acidification.
- Robinson, Deena, and Martina Igini. 2025. “15 Biggest Environmental Problems of 2025.” Earth.org. Earth.org. January 9, 2025. https://earth.org/the-biggest-environmental-problems-of-our-lifetime/.
- Sosdian, S. M., R. Greenop, M. P. Hain, G. L. Foster, P. N. Pearson, and C. H. Lear. 2018. “Constraining the Evolution of Neogene Ocean Carbonate Chemistry Using the Boron Isotope PH Proxy.” Earth and Planetary Science Letters 498 (September): 362–76. https://doi.org/10.1016/j.epsl.2018.06.017.


